Sustainable Discovery and Development of Antibiotics

Sustainable Discovery and Development of Antibiotics
The introduction of penicillin transformed the practice of medicine and contributed to mortality from infections plummeting by about 80% in the United States, from 280 to 60 per 100,000 population.1This transformation is now under threat, however, as rising rates of antibiotic resistance are leading society toward what some experts have warned will be a post-antibiotic era.2,3 Advocacy efforts have led to the establishment of “push” economic incentives that support the discovery and development of new antibiotics. Such incentives include grants and contracts from government agencies (e.g., the National Institutes of Health and the Biomedical Advanced Research and Development Authority) and public–private partnerships (e.g., CARB-X [Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator] and ND4BB [New Drugs for Bad Bugs]).
Economic incentives have collectively helped catalyze a marked increase in development. In March 2019, the Pew Charitable Trusts identified 42 antibiotics in clinical and preclinical development; 6 were in clinical development in 2004.1 After decreasing by more than 90% between 1983 and 2012, the number of new antibiotics approved each year by the Food and Drug Administration (FDA) has tripled in the past 6 years.
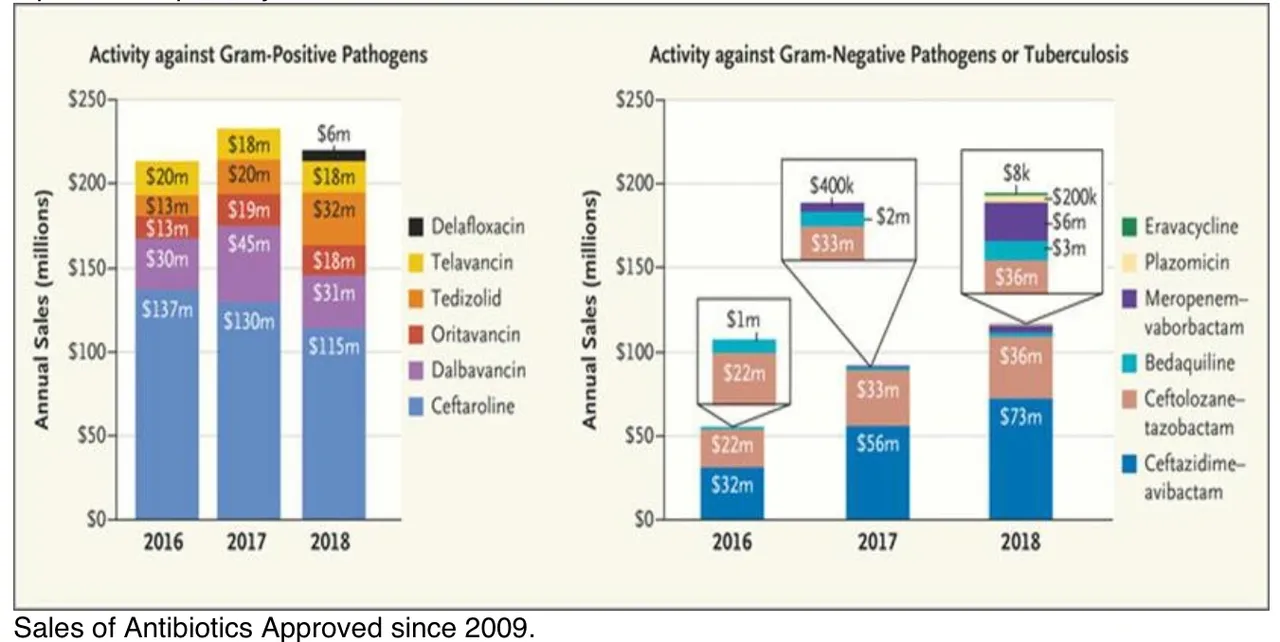
But there is a dark lining to this silver cloud. The majority of antibiotics that are newly approved or in development don’t help meet the critical need for new oral and parenteral drugs that are effective against extremely drug-resistant (XDR) gram-negative bacilli and mycobacterial infections. Moreover, several recently approved antibiotics targeting gram-negative bacilli, as well as many others in clinical development, are redundant, since they target the same resistant organism. The four antibiotics approved since 2015 that target XDR gram-negative bacilli (ceftazidime–avibactam, meropenem–vaborbactam, plazomicin, and eravacycline) are all active against carbapenem-resistant Enterobacteriaceae. As a result, these antibiotics compete with each other for market share, which has led to poor sales (see graph).
Indeed, all antibiotics approved in the past decade have had disappointing sales, triggering renewed threats of companies exiting the antibiotics business. In an ominous sign, Achaogen filed for bankruptcy in April 2019, despite having received FDA approval for plazomicin.
Rising rates of resistance appear to create new market opportunities for antibiotics. However, the absolute number of infections caused by each type of resistant bacterium is relatively small. Each newly approved antibiotic thus captures an ever-shrinking share of an increasingly splintered market — a problem that will only worsen over time. Short treatment durations and a coordinated program of antibiotic stewardship also contribute to low sales.1
One proposed solution is to establish additional publicly funded economic incentives to promote antibiotics research and development.2 Yet existing incentives have failed to trigger consistent development of the most-needed antibiotics. Furthermore, an estimated $1 billion to $2 billion in new incentives would be required for each successfully developed antibiotic.2 New antibiotics will continually be needed as the emergence of resistance erodes each drug’s effectiveness for treating at least some types of bacteria.3 Relying on new incentives to ensure sustainable antibiotic efficacy would therefore require billions of dollars in new incentives in perpetuity.
In 2016, British economist Jim O’Neill authored an influential report proposing improved economic incentives to lure companies back into antibiotics research and development.2 Three years later, however, he conceded that it may be time to “just take it away from them and take it over.”4
Indeed, it may well be. We believe that the current entrepreneurial development model for antibiotics is broken and needs to be fundamentally transformed. An improved system would encourage discovery and development of truly needed antibiotics that improve patient outcomes, rather than continued development of “me too” drugs; would make development of antibiotics with low peak sales economically feasible; and would permit more effective control over the postapproval use of antibiotics in order to prolong their effectiveness.
In these regards, nonprofit organizations have substantial advantages over for-profit companies.1,5Nonprofits can eschew opportunities to enter larger markets in favor of addressing unmet needs because they don’t face pressure to generate continuous revenue growth to drive up shareholder value — a problem that is exacerbated for small companies, whose venture-capitalist investors demand very high rates of return over short periods. Because they lack shareholders, nonprofits also face less pressure to increase drug prices and are better positioned to control postapproval antibiotic use (e.g., through the existing limited-population antibiotic drug regulatory pathway). A drug with annual sales in the tens of millions of dollars is a catastrophic failure for many for-profit companies but would be a lifeline for nonprofits, which could reinvest revenue from the drug to sustain research and development efforts. Organizations that highlight the potential of nonprofits in this area include the TB Alliance, which has participated in the development of new antibiotics for tuberculosis, and the Medicines for Malaria Venture, which developed artesunate and is actively developing other antimalarials.5
Nonprofit organizations could bring antibiotics to the market themselves, or they could license or sell compounds that have been through initial testing or early-stage clinical trials to for-profit partners that could then carry such products through late-stage development. If for-profit companies were not involved until later in the drug-development process, the risk of early failure taken on by such companies would be substantially reduced, up-front research and development costs would be covered by other entities, and discounting of future sales would be minimized.
For such a strategy to be effective, nonprofit development of antibiotics would have to be part of a broader framework of new ways to combat infections. Potential approaches could include better targeting of existing financial incentives for development of antibiotics and finding strategies for preventing or treating infections that don’t rely solely on antibiotics.1 Nonprofit organizations could participate in developing new vaccines to prevent infections, as well as immunotherapies, nutritional-deprivation strategies, inflammatory modulators, and other approaches to treat them.3
The greatest challenge associated with a nonprofit-driven model is identifying seed capital for establishing such organizations. In this regard, we believe that making a one-time investment of a billion dollars to create several new nonprofits that sustainably discover and develop new antibiotics might be a better long-term investment than perpetually offering multibillion-dollar prizes or other pull incentives for each new antibiotic.
Shifting to a new model of drug development will naturally threaten players with vested interests in for-profit discovery of antibiotics. Traditionalists will probably argue that nonprofits cannot replace for-profit industry as a vehicle for innovation. But for-profit companies haven’t been able to reliably generate sufficient income from the sale of new antibiotics to satisfy shareholder demands for revenue growth, despite frequently focusing their efforts on antibiotics with larger perceived markets at the expense of addressing unmet needs.
The economic outlook for development of antibiotics will worsen over time as new ones reach the clinic and contribute to an ever-more commoditized market. The increasingly loud drumbeat calling for additional subsidies for the pharmaceutical industry to develop new antibiotics conflicts with the realities of the daunting U.S. federal debt that has been driven up by high health care costs, the low esteem in which the public holds the pharmaceutical industry, and rising concerns about the costs of pharmaceuticals. Such dynamics will impede policies that include new pharmaceutical subsidies, irrespective of their potential effectiveness.
An alternative model for sustaining discovery of antibiotics is overdue. We believe it is time to seriously consider the establishment of nonprofit organizations for developing these lifesaving drugs.
Disclosure forms provided by the authors are available at NEJM.org.
This article was published on June 19, 2019, and updated on July 24, 2019, at NEJM.org.
Author Affiliations
From the Stritch School of Medicine and the Parkinson School of Health Sciences and Public Health, Loyola University Chicago, Maywood, IL (T.B.N.); the David Geffen School of Medicine at the University of California, Los Angeles (E.P.B.) and the Los Angeles County and University of Southern California Medical Center (B.S.) — both in Los Angeles; the Providence Portland Medical Center and University of Oregon Health Sciences School of Medicine, Portland (D.N.G.); and the Johns Hopkins University School of Medicine, Baltimore (J.G.B.).
Supplementary Material
136KB
References (5)
- 1. Spellberg B. The antibacterial pipeline: why is it drying up, and what must be done about it? In: Institute of Medicine. Antibiotic resistance: implications for global health and novel intervention strategies: workshop summary. Washington, DC: National Academies Press, 2010:326-364 (https://www.nap.edu/catalog/12925/antibiotic-resistance-implications-for-global-health-and-novel-intervention-strategies).
- 2. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. London: Review on Antimicrobial Resistance, May 2016 (https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf).
- 3.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med 2013;368:299-302.
- 4. Gallagher J. Take over pharma to create new medicines, says top adviser. BBC News. March 27, 2019 (https://www.bbc.com/news/health-47719269).
- 5.Hale VG, Woo K, Lipton HL. Oxymoron no more: the potential of nonprofit drug companies to deliver on the promise of medicines for the developing world. Health Aff (Millwood) 2005;24:1057-1063.
Disclaimer: Any third-party material in this email has been shared under fair use provisions of U.S. copyright law, without further verification of its accuracy/veracity. It does not necessarily represent my views nor those of NIAID, NIH, HHS, or the U.S. government.
